Anthelban V
Product Type: PRESCRIPTION ANIMAL DRUG LABEL
Autor Name: Teva Animal Health, Inc.
Code Source: 59130-692
Route of Administration: ORAL
ANADA 200-246, Approved by FDA
ANTHELBAN ® V
(pyrantel pamoate)
Equine Anthelmintic Suspension
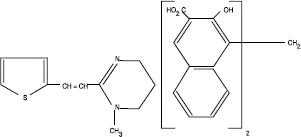
Pyrantel pamoate is a compound belonging to a family classified chemically as tetrahydropyrimidines. It is a yellow, water-insoluble crystalline salt of the tetrahydropyrimidine base and pamoic acid containing 34.7% base activity. The chemical structure and name are given below:
(E)-1,4,5,6-Tetrahydro-1-methyl-2-[2-(2-thienyl) vinyl] pyrimidine 4, 4' methylenebis [3-hydroxy-2-naphthoate] (1:1)
Chemical Structure
Stomach Tube - Measure the appropriate dosage of Anthelban ® V and mix in the desired quantity of water. Protect drench from direct sunlight and administer to the animal immediately following mixing. Do not attempt to store diluted suspension.
Anthelban ® V is inactive against the common horse bot ( Gasterophilus spp .). However, Anthelban ® V may be administered concurrently with carbon disulfide observing the usual precautions with carbon disulfide.
Dose Syringe - Draw the appropriate dosage of Anthelban ® V into a dose syringe and administer to the animal. Do not expose Anthelban® V to direct sunlight.
Feed - Mix the appropriate dosage of Anthelban ® V in the normal grain ration. Fasting of animals prior to or following treatment is not required.
EFFICACY
Critical (worm-count) studies in horses demonstrated that pyrantel pamoate administered at the recommended dosage was efficacious against mature infections of Strongylus vulgaris ( > 90%), S. edentatus (69%), S. equinus ( > 90%), Oxyuris equi (81%), Parascaris equorum ( > 90%), and small strongyles (90%).
Manufactured by
Teva Animal Health, Inc.
St. Joseph, MO 64503
Trademarks are property of Teva Animal Health, Inc.
Take Time Observe Label Directions

Anthelban V Pint Label

Anthelban V Pint Booklet

Anthelban V Quart Label

Anthelban V 1 Quart Booklet

Autor Name: Teva Animal Health, Inc.
Code Source: 59130-692
Route of Administration: ORAL
Anthelban V
Generic: Pyrantel PamoateIngredients:
- PYRANTEL PAMOATE PYRANTEL : Active ingredient - moiety is basis of strength - 50 mg in 1 mL
Package Description:
- 473 mL in 1 BOTTLE, PLASTIC
- 946 mL in 1 BOTTLE, PLASTIC
ANADA 200-246, Approved by FDA
ANTHELBAN ® V
(pyrantel pamoate)
Equine Anthelmintic Suspension
CAUTIONS:
Federal law restricts this drug to use by or on the order of a licensed veterinarian.DESCRIPTION
Anthelban ® V is a suspension of pyrantel pamoate in a palatable vanilla-flavored vehicle. Each mL contains 50 mg of pyrantel base as pyrantel pamoate.Pyrantel pamoate is a compound belonging to a family classified chemically as tetrahydropyrimidines. It is a yellow, water-insoluble crystalline salt of the tetrahydropyrimidine base and pamoic acid containing 34.7% base activity. The chemical structure and name are given below:
(E)-1,4,5,6-Tetrahydro-1-methyl-2-[2-(2-thienyl) vinyl] pyrimidine 4, 4' methylenebis [3-hydroxy-2-naphthoate] (1:1)
Chemical Structure

INDICATIONS AND USAGE
For the removal and control of mature infections of large strongyles ( Strongylus vulgaris, S. edentatus, S. equinus ); pinworms ( Oxyuris equi ); large roundworms ( Parascaris equorum ); and small strongyles in horses and ponies.CONTRAINDICATIONS
It is recommended that severely debilitated animals not be treated with this preparation.WARNINGS
Do not use in horses intended for human consumption. Keep out of reach of children.PRECAUTION
This product is a suspension and as such will separate. To insure uniform resuspension and to achieve proper dosage, it is extremely important that the product be shaken and stirred thoroughly before every use.DOSAGE AND ADMINISTRATION
Administer 3 mg pyrantel base per lb of body weight (6 mL Anthelban ® V per 100 lb of body weight). It is recommended that severely debilitated animals not be treated with this preparation. For maximum control of parasitism, it is recommended that foals (2-8 months of age) be dosed every 4 weeks. To minimize potential hazard that the mare may pose to the foal, she should be treated 1 month prior to anticipated foaling date followed by retreatment 10 days to 2 weeks after birth of foal. Horses over 8 months of age should be routinely dosed every 6 weeks.DIRECTIONS FOR USE
Anthelban ® V may be administered by means of a stomach tube, dose syringe or by mixing into the feed.Stomach Tube - Measure the appropriate dosage of Anthelban ® V and mix in the desired quantity of water. Protect drench from direct sunlight and administer to the animal immediately following mixing. Do not attempt to store diluted suspension.
Anthelban ® V is inactive against the common horse bot ( Gasterophilus spp .). However, Anthelban ® V may be administered concurrently with carbon disulfide observing the usual precautions with carbon disulfide.
Dose Syringe - Draw the appropriate dosage of Anthelban ® V into a dose syringe and administer to the animal. Do not expose Anthelban® V to direct sunlight.
Feed - Mix the appropriate dosage of Anthelban ® V in the normal grain ration. Fasting of animals prior to or following treatment is not required.
EFFICACY
Critical (worm-count) studies in horses demonstrated that pyrantel pamoate administered at the recommended dosage was efficacious against mature infections of Strongylus vulgaris ( > 90%), S. edentatus (69%), S. equinus ( > 90%), Oxyuris equi (81%), Parascaris equorum ( > 90%), and small strongyles (90%).
SAFETY
Pyrantel pamoate is well tolerated by horses and ponies of all ages. No adverse drug response was observed when dose rates up to 60 mg of pyrantel base per lb of body weight were administered by stomach tube nor when 3 mg base per lb was given by intratracheal injection. The reproductive performance of pregnant mares and stud horses dosed with pyrantel pamoate has not been affected.STORAGE
Store at controlled room temperature 20 °-25°C (68°-77°F).Manufactured by
Teva Animal Health, Inc.
St. Joseph, MO 64503
Trademarks are property of Teva Animal Health, Inc.
Take Time Observe Label Directions

PRINCIPAL DISPLAY PANEL
Anthelban V Pint Label

PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Anthelban V Pint Booklet

PRINCIPAL DISPLAY PANEL
Anthelban V Quart Label

PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Anthelban V 1 Quart Booklet

