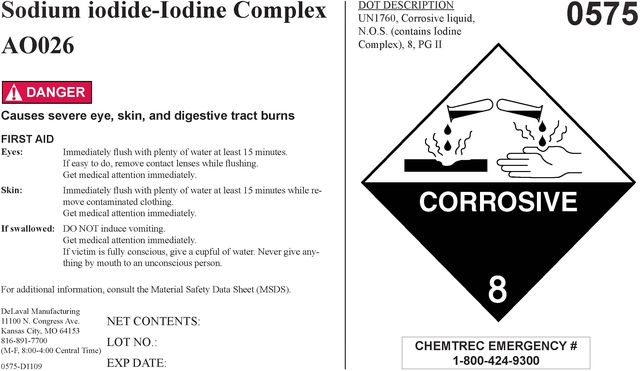
Sodium iodide-Iodine Complex A0026
Product Type: OTC ANIMAL DRUG LABEL
Autor Name: WestAgro, Inc.
Code Source: 33392-575
Route of Administration: TOPICAL
! DANGER
Causes severe eye, skin, and digestive tract burns
FIRST AID Eyes: Immediately flush with plenty of water at least 15 minutes. If easy to do, remove contact lenses while flushing. Get medical attention immediately.
Skin: Immediately flush with plenty of water at least 15 minutes while removing contaminated clothing. Get medical attention immediately.
If swallowed: DO NOT induce vomiting. Get medical attention immediately. If victim is fully conscious, give a cupful of water. Never give anything my mouth to an unconscious person.
For additional information, consult the Material Safety Data Sheet (MSDS).
DeLaval Manufacturing
11100 N. Congress Ave.
Kansas City, MO 64153
816-891-7700
(M-F, 8:00-4:00 Central Time)
0575-D1109
NET CONTENTS:
LOT NO.:
EXP DATE:
DOT DESCRIPTION
UN1760, Corrosive Liquid,
N.O.S. contains Iodine
Complex), 8, PG II
0575
CORROSIVE 8
CHEMTREC EMERGENCY NO. 1-800-424-9300
Product Container Label
Autor Name: WestAgro, Inc.
Code Source: 33392-575
Route of Administration: TOPICAL
Sodium iodide-Iodine Complex
Generic: IodineIngredients:
- Iodine Iodine : Active ingredient - basis of strength - 1.37 kg in 1 L
- Sodium iodide : Inactive ingredient
- Water : Inactive ingredient
Package Description:
- 99.3 L in 1 DRUM
! DANGER
Causes severe eye, skin, and digestive tract burns
FIRST AID Eyes: Immediately flush with plenty of water at least 15 minutes. If easy to do, remove contact lenses while flushing. Get medical attention immediately.
Skin: Immediately flush with plenty of water at least 15 minutes while removing contaminated clothing. Get medical attention immediately.
If swallowed: DO NOT induce vomiting. Get medical attention immediately. If victim is fully conscious, give a cupful of water. Never give anything my mouth to an unconscious person.
For additional information, consult the Material Safety Data Sheet (MSDS).
DeLaval Manufacturing
11100 N. Congress Ave.
Kansas City, MO 64153
816-891-7700
(M-F, 8:00-4:00 Central Time)
0575-D1109
NET CONTENTS:
LOT NO.:
EXP DATE:
DOT DESCRIPTION
UN1760, Corrosive Liquid,
N.O.S. contains Iodine
Complex), 8, PG II
0575
CORROSIVE 8
CHEMTREC EMERGENCY NO. 1-800-424-9300
Product Container Label

