Miconosol Lotion 1%
Product Type: PRESCRIPTION ANIMAL DRUG LABEL
Autor Name: Med-Pharmex, Inc
Code Source: 54925-031
Route of Administration: TOPICAL
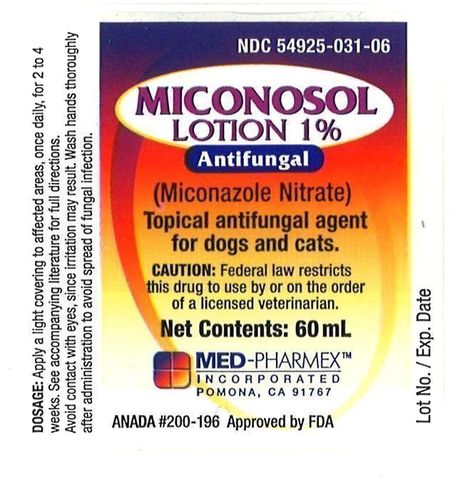
Apply a light covering of MICONAZOLE NITRATE Lotion to affected areas, once daily, for 2 to 4 weeks. Application is best accomplished using a gauze pad or cotton swab. Medication must be continued until the infecting organism is completely eradicated as indicated by appropriate clinical or laboratory examination. If no improvement is noticed within 2 weeks, diagnosis should be re-evaluated. Difficult cases may require treatment for 6 weeks.
General measures in regard to hygiene should be observed to control sources of infection or reinfection. Clipping of hair around and over the sites of infection should be done at the start of treatment and again as necessary.
Miconosol Lotion 1%
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Miconazole Lotion Insert.

Miconosol Lotion 60mL Label
Autor Name: Med-Pharmex, Inc
Code Source: 54925-031
Route of Administration: TOPICAL
Miconosol
Generic: Miconazole NitrateIngredients:
- Miconazole Nitrate Miconazole : Active ingredient - basis of strength - 1.0 g in 100 mL
- ALCOHOL : Inactive ingredient
- POLYETHYLENE GLYCOL 400 : Inactive ingredient
Package Description:
- 60 mL in 1 BOTTLE
Description
MICONAZOLE NITRATE lotion is a synthetic antifungal agent for use in dogs and cats. It contains:1.15% miconazole nitrate (equivalent to 1% miconazole base by weight). polyethylene glycol 400 and ethyl alcohol 55%.Indications
MICONAZOLE NITRATE Lotion is indicated for the treatment of fungal lnfections in dogs and cats caused by Microsporum canis, Microsporum gypseum and Trichophyton mentagrophytes.Precautions
In the event of sensitization or irritation due to MICONAZOLE NITRATE Lotion, treatment should be discontinued. Avoid contact with eyes, since irritation may result. Wash hands thoroughly after administration to avoid spread of fungal infection.Dosage and Administration
Accurate diagnosis of the infecting organism is essential. ldentification should be made either by direct microscopic examination of a mounting of infected tissue in a solution of potassium hydroxide, or by culture on an appropriate medium.Apply a light covering of MICONAZOLE NITRATE Lotion to affected areas, once daily, for 2 to 4 weeks. Application is best accomplished using a gauze pad or cotton swab. Medication must be continued until the infecting organism is completely eradicated as indicated by appropriate clinical or laboratory examination. If no improvement is noticed within 2 weeks, diagnosis should be re-evaluated. Difficult cases may require treatment for 6 weeks.
General measures in regard to hygiene should be observed to control sources of infection or reinfection. Clipping of hair around and over the sites of infection should be done at the start of treatment and again as necessary.
How Supplied
M1CONAZOLE NITRATE Lotion in 60 mL containers.Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.Miconosol Lotion 1%
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Miconazole Lotion Insert.
Miconosol Lotion 60mL Label
