BM 5000 UC
Product Type: OTC ANIMAL DRUG LABEL
Autor Name: BouMatic, LLC
Code Source: 48106-1171
Route of Administration: TOPICAL
HAZARD: Corrosive. Contains iodine. Severe eye, skin and digestive tract irritant. May cause permanent eye damage.
PRECAUTION: Avoid eye and skin contact. Do not breathe mist or spray. Wear impermeable gloves, safety glasses, rubber boots, rubber apron, and face shield. Use only in a well ventilated area.
FIRST AID: If in contact with eyes, flush with water for at least 15 minutes. Contact a physician immediately. If in contact with skin, wash well with water. Contact a physician. If ingested, drink large volumes of water. Do not induce vomiting. Contact a physician immediately. If breathing difficulty occurs, move to fresh air. Contact a physician.
SEE MATERIAL SAFETY DATA SHEET
BM 5000 UC label
Blank Label
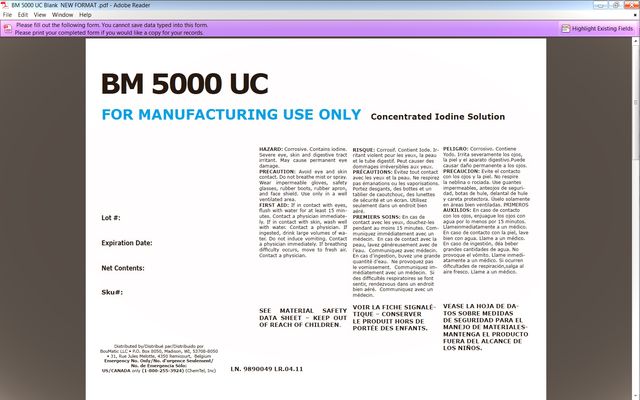
FOR MANUFACTURING USE ONLY
Concentrated Iodine Solution
Autor Name: BouMatic, LLC
Code Source: 48106-1171
Route of Administration: TOPICAL
BM 5000 UC
Generic: IODINEIngredients:
- IODINE IODINE : Active ingredient - basis of strength - 550 mL in 10 L
Package Description:
- 1040 L in 1 CONTAINER, FLEXIBLE INTERMEDIATE BULK
HAZARD: Corrosive. Contains iodine. Severe eye, skin and digestive tract irritant. May cause permanent eye damage.
PRECAUTION: Avoid eye and skin contact. Do not breathe mist or spray. Wear impermeable gloves, safety glasses, rubber boots, rubber apron, and face shield. Use only in a well ventilated area.
FIRST AID: If in contact with eyes, flush with water for at least 15 minutes. Contact a physician immediately. If in contact with skin, wash well with water. Contact a physician. If ingested, drink large volumes of water. Do not induce vomiting. Contact a physician immediately. If breathing difficulty occurs, move to fresh air. Contact a physician.
SEE MATERIAL SAFETY DATA SHEET
BM 5000 UC label
Blank Label

FOR MANUFACTURING USE ONLY
Concentrated Iodine Solution
