intervet safe-guard® (fenbendazole)
Product Type: OTC ANIMAL DRUG LABEL
Autor Name: Schering Corporation
Code Source: 57926-088
Route of Administration: ORAL
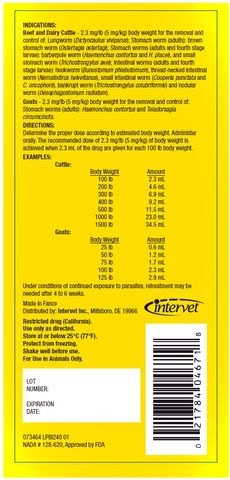
Goats - 2.3 mg/lb (5 mg/kg) body weight for the removal and control of: Stomach worms (adults): Haemonchus contortus and Teladorsagia circumcincta .
Restricted drug (California).
Use only as directed.
Store at or below 25 °C (77°F).
Protect from freezing.
Shake well before use.
For Use in Animals Only.
RESIDUE WARNINGS: Cattle must not be slaughtered within 8 days following treatment. A withdrawal period has not been established for this product in pre-ruminating calves. Do not use in calves to be processed for veal. Goats must not be slaughtered for food within 6 days following treatment. Because a withdrawal time in milk has not been established, do not use in lactating goats. For dairy cattle, there is no milk withdrawal period.
Made in Fance
Distributed by: Intervet Inc. , Millsboro, DE 19966
NADA # 128-620, Approved by FDA
safe-guard ®
(fenbendazole)
Dewormer
for Beef & Dairy Cattle and Goats
Suspension 10% (100 mg/mL)
RESIDUE WARNINGS: Cattle must not be slaughtered within 8 days following treatment. A withdrawal period has not been established for this product in pre-ruminat- ing calves. Do not use in calves to be processed for veal. Goats must not be slaughtered for food within 6 days following treatment. Because a withdrawal time in milk has not been established, do not use in lactating goats. For dairy cattle, there is no milk withdrawal period.
Consult your veterinarian for assistance in the diagnosis,
treatment and control of parasitism.
Keep this and all medication out of the reach of children.
1,000 mL (33.8 fl oz)
093241 LPFI240 01
Principal Display Panel - 1,000 mL label

Principal Display Panel - 1,000 mL label
Autor Name: Schering Corporation
Code Source: 57926-088
Route of Administration: ORAL
Safe-Guard
Generic: FenbendazoleIngredients:
- Fenbendazole Fenbendazole : Active ingredient - basis of strength - 100 mg in 1 mL
- methylparaben : Inactive ingredient
- propylparaben : Inactive ingredient
- silicon dioxide : Inactive ingredient
- carboxymethylcellulose sodium : Inactive ingredient
- povidones : Inactive ingredient
- trisodium citrate dihydrate : Inactive ingredient
- citric acid monohydrate : Inactive ingredient
- water : Inactive ingredient
Package Description:
- 1000 mL in 1 BOTTLE
INDICATIONS
Beef and Dairy Cattle - 2.3 mg/lb (5 mg/kg) body weight for the removal and control of: Lungworm ( Dictyocaulus viviparus ); Stomach worm (adults): brown stomach worm ( Ostertagia ostertagi ); Stomach worms (adults and fourth stage larvae): barberpole worm ( Haemonchus contortus and H. placei ), and small stomach worm ( Trichostrongylus axei ); Intestinal worms (adults and fourth stage larvae): hookworm ( Bunostomum phlebotomum ), thread-necked intestinal worm ( Nematodirus helvetianus ), small intestinal worm ( Cooperia punctata and C. oncophora ), bankrupt worm ( Trichostrongylus colubriformis ) and nodular worm ( Oesophagostomum radiatum ).Goats - 2.3 mg/lb (5 mg/kg) body weight for the removal and control of: Stomach worms (adults): Haemonchus contortus and Teladorsagia circumcincta .
DIRECTIONS
Determine the proper dose according to estimated body weight. Administer orally. The recommended dose of 2.3 mg/lb (5 mg/kg) of body weight is achieved when 2.3 mL of the drug are given for each 100 lb body weight.EXAMPLES
Under conditions of continued exposure to parasites, retreatment may be needed after 4 to 6 weeks.Restricted drug (California).
Use only as directed.
Store at or below 25 °C (77°F).
Protect from freezing.
Shake well before use.
For Use in Animals Only.
RESIDUE WARNINGS: Cattle must not be slaughtered within 8 days following treatment. A withdrawal period has not been established for this product in pre-ruminating calves. Do not use in calves to be processed for veal. Goats must not be slaughtered for food within 6 days following treatment. Because a withdrawal time in milk has not been established, do not use in lactating goats. For dairy cattle, there is no milk withdrawal period.
Made in Fance
Distributed by: Intervet Inc. , Millsboro, DE 19966
NADA # 128-620, Approved by FDA
PRINCIPAL DISPLAY PANEL - 1,000 mL label
intervetsafe-guard ®
(fenbendazole)
Dewormer
for Beef & Dairy Cattle and Goats
Suspension 10% (100 mg/mL)
RESIDUE WARNINGS: Cattle must not be slaughtered within 8 days following treatment. A withdrawal period has not been established for this product in pre-ruminat- ing calves. Do not use in calves to be processed for veal. Goats must not be slaughtered for food within 6 days following treatment. Because a withdrawal time in milk has not been established, do not use in lactating goats. For dairy cattle, there is no milk withdrawal period.
Consult your veterinarian for assistance in the diagnosis,
treatment and control of parasitism.
Keep this and all medication out of the reach of children.
1,000 mL (33.8 fl oz)
093241 LPFI240 01
Principal Display Panel - 1,000 mL label

Principal Display Panel - 1,000 mL label

